
In recent years, the landscape of clinical research has undergone a significant transformation with the rise of decentralized clinical trials (DCTs).
Unlike traditional clinical trials that require patients to visit centralized study sites, DCTs leverage digital technologies to enable remote participation, making the process more accessible, efficient, and patient-centric. From telemedicine and wearable devices to electronic data capture and remote monitoring, DCTs integrate modern tools to streamline trial operations, enhance data quality, and broaden patient reach.
Let’s explore the key benefits of decentralized clinical trials (DCTs), organizational strategies, enabling technologies, challenges & solutions and FDA guidelines to better understand this innovative approach to modern clinical research
What Are Decentralized Clinical Trials?
Decentralized clinical trials (DCTs) are a type of clinical trial that uses digital technologies and remote methods to conduct clinical research. Unlike traditional clinical trials, which require participants to travel to a study site to receive treatment and be monitored by healthcare professionals, DCTs allow patients to participate in the trial from the comfort of their homes or other locations.In DCTs, data collection, monitoring, and analysis are done remotely using digital tools, such as smartphones, wearable devices, and telemedicine platforms.
Benefits of Decentralized Clinical Trials
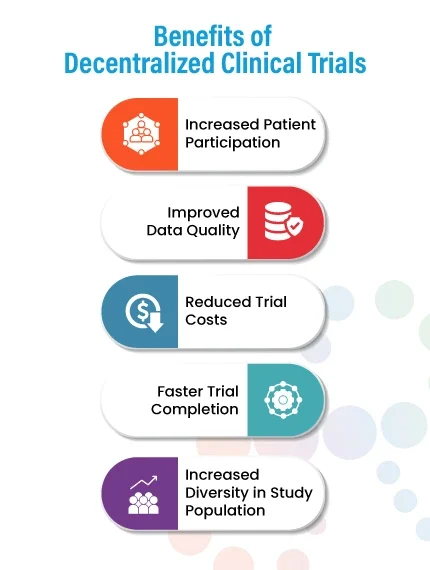
- Increased Patient Participation:
DCTs allow patients to participate in clinical trials from their homes, local clinics, or pharmacies, making it easier for them to participate in the study. This can increase enrolment and retention in the trial, as patients do not have to travel long distances or take time off from work to participate. - Improved Data Quality:
DCTs can improve data quality by reducing the risk of missing data, data transcription errors, and data entry errors. DCTs can also provide real-time data monitoring, enabling early detection of safety issues and improving the overall quality of data collected. - Reduced Trial Costs:
DCTs can help to reduce the overall cost of conducting clinical trials by eliminating or reducing the need for physical trial sites, transportation and accommodation expenses, and site monitoring costs. - Faster Trial Completion:
DCTs can lead to faster completion of clinical trials by reducing the time required for patient recruitment, monitoring, and data collection and reducing the time needed for regulatory approval. - Increased Diversity in Study Population: DCTs can enable a more diverse study population by overcoming geographical barriers and making it easier for patients with mobility or transportation limitations to participate in the study. This can lead to a more representative study population and more generalizable study results. Overall, decentralized clinical trials can provide numerous benefits, both for patients and for trial sponsors, and are likely to become more widely used in the future.
How is a decentralized clinical study organized?
Conducting decentralized or remote clinical trials requires careful planning, coordination, and implementation of technology to enable remote participation. Some key elements in organizing a decentralized study include:
- Home Health Care
Local home health care providers assist with basic in-person procedures and sample collection. - Lab Drawings Rather than visit designated labs, samples are collected by home nurses or at local facilities.
- Shipments of investigational products (IP) made directly to patients (DTP)
Medications are shipped directly to patient homes from central pharmacies. - Off-Site Inspection
Monitoring teams perform remote source data verification rather than on-site monitoring. from various sources are compiled and monitored centrally in real-time. - Update study plans and protocols Existing protocols may need adjustment for remote procedures and decentralization.
- The assessment of the risk
Sponsors and contract research organizations must thoroughly assess the risks associated with remote procedures and monitoring. Additional quality control and training measures may need to be implemented to ensure high quality data collection. - Study logistics and patient focus
Logistics must be optimized for direct-to-patient shipment of study materials and remote coordination of study visits. There is a greater focus on patient convenience and experience. - Remote approval
Regulators must approve remote electronic consent procedures, at home self-administration of study drugs, and remote data collection. - Online applications and recruitment procedures
Participants can learn about trials and apply online. Screening and enrolment procedures can also be conducted remotely via online platforms. - Use of telemedicine
Virtual video visits replace some traditional in-person assessments. Remote monitoring devices allow continuous data collection.

Accelerate Your Clinical Trials with Full-Service CRO & Tech-Enabled Solutions
Partner with Clinvigilant to streamline every phase of your study-from patient recruitment and site management to CTMS, eTMF, EDC, and remote monitoring. Our end-to-end CRO services are designed for speed, compliance, and stronger patient engagement.
Platform or Technology Used in Decentralized Trials
Decentralized clinical trials (DCTs) rely heavily on digital technologies for remote data collection, patient monitoring, and communication between patients, investigators, and study coordinators. Some of the commonly used technologies in DCTs include:
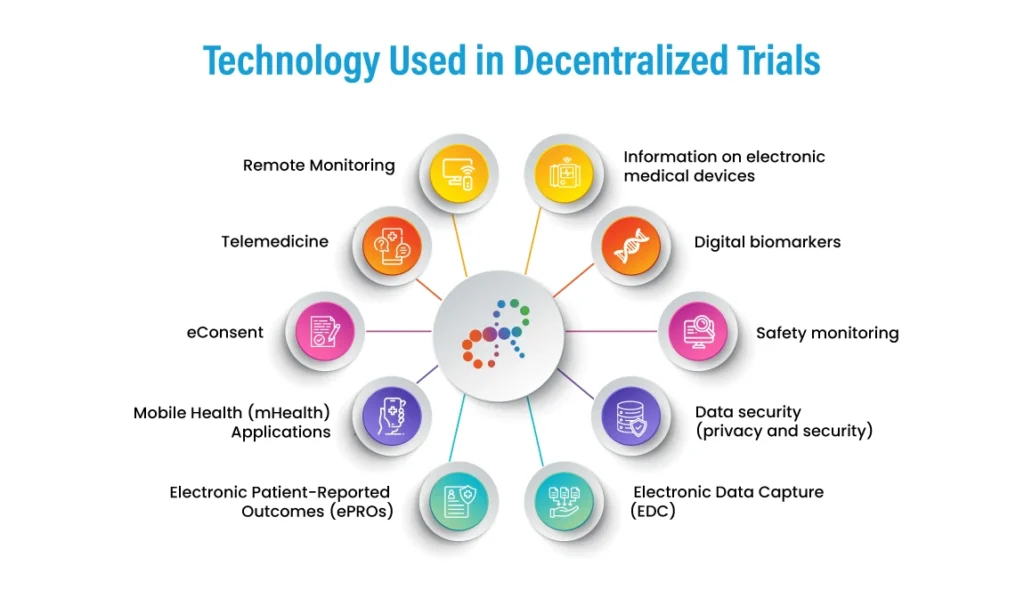
- Information on electronic medical devices
Connected devices can remotely monitor key metrics like blood pressure, heart rate, glucose levels and more. - Digital biomarkers
Digital apps and connected devices assess indicators like movement, cognitive function, and sleep quality. - Safety monitoring
Wearables and at-home medical devices allow continuous safety monitoring. - Data security (privacy and security)
End-to-end data encryption protects sensitive patient information. Advanced cybersecurity measures must be implemented. - Electronic Data Capture (EDC)
EDC is a technology that enables clinical trial data to be collected, stored, and managed electronically. EDC can improve data quality and reduce the risk of transcription errors, and can also enable real-time data monitoring. - Remote Monitoring
Remote monitoring involves using digital technologies, such as wearable devices or mobile applications, to monitor patient health and behaviour remotely. Remote monitoring can provide real-time patient outcomes data and enable early detection of safety issues.
- Telemedicine
Telemedicine involves video conferencing and other digital technologies to enable remote consultations between patients and healthcare providers. Telemedicine can improve patient access to healthcare and reduce patients’ need to travel to physical trial sites. - eConsent
eConsent involves using digital technologies, such as electronic signatures or interactive multimedia, to obtain informed consent from study participants. eConsent can improve the efficiency and accuracy of the consent process and patient understanding of the study. - Mobile Health (mHealth) Applications
mHealth applications are designed to support patient self-management and remote monitoring. mHealth applications can give patients real-time feedback on their health status and enable them to track their health outcomes. - Electronic Patient-Reported Outcomes (ePROs)
ePROs are digital tools for collecting patient-reported outcomes data. ePROs can improve the accuracy and completeness of patient-reported data and reduce the need for in-person visits for data collection.
Selecting the right decentralized clinical trials platform is critical for seamless integration of devices, patient apps, data capture tools, and telemedicine services.
Guidelines for Implementing Decentralized Clinical Trials
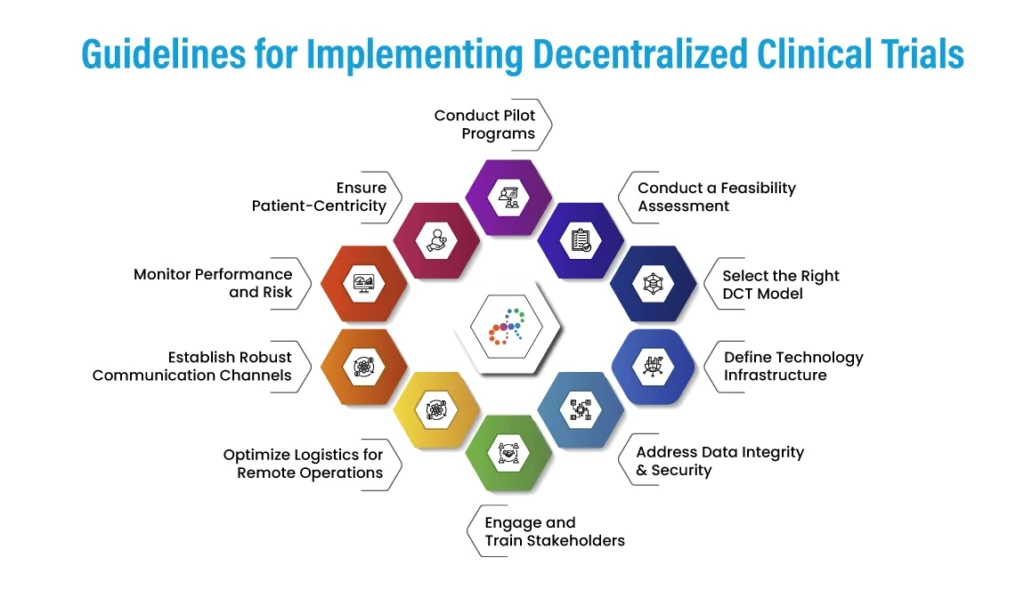
- Conduct a Feasibility Assessment
- Evaluate protocol fit: Not all trials are suitable for decentralization. Assess based on disease area, patient population, study complexity, and data collection needs.
- Regulatory landscape: Review local and global regulations (e.g., FDA, EMA, ICH) to ensure compliance in all operating geographies.
- Stakeholder readiness: Gauge readiness across sponsors, sites, investigators, and patients.
-
Select the Right DCT Model
- Hybrid Model: Combine on-site visits with remote monitoring.
- Fully Decentralized Model: All trial components are managed remotely.
- Choose based on:
- Trial phase
- Complexity of endpoints
- Patient safety and oversight requirements
-
Define Technology Infrastructure
- eConsent: Implement remote informed consent platforms compliant with GCP and 21 CFR Part 11.
- ePRO/eCOA: Use electronic patient-reported outcomes tools for real-time symptom and compliance tracking.
- Wearables & sensors: Integrate IoT devices for continuous remote monitoring.
- Telehealth platforms: Enable virtual visits, consultations, and assessments.
- RTSM & EDC: Use real-time randomization and electronic data capture systems to maintain study integrity.
-
Address Data Integrity & Security
- Data privacy: Ensure compliance with HIPAA, GDPR, and local data protection laws.
- Audit trails: Maintain traceability in all digital systems.
- Secure transmission: Use encrypted, validated platforms for all data sharing.
-
Engage and Train Stakeholders
- Patients:
- Offer onboarding support for digital tools.
- Provide clear instructions and tech support.
- Sites and Investigators:
- Train on protocol amendments, virtual assessments, and remote source data verification (rSDV).
- Monitors & CRAs:
- Equip them to manage centralized or risk-based monitoring remotely.
- Patients:
- Optimize Logistics for Remote Operations
- Home health services: Partner with mobile nursing providers for in-home assessments.
- Direct-to-patient (DTP) shipments: Set up a supply chain for investigational products (IPs) and sample collection kits.
- Remote sample collection: Utilize certified labs with patient pickup/drop-off or mail-in options.
-
Establish Robust Communication Channels
- 24/7 support lines: For patients and sites.
- Integrated communication tools: For seamless coordination among sponsors, CROs, and sites.
- Regular virtual check-ins: To maintain engagement and study compliance.
-
Monitor Performance and Risk
- Centralized monitoring: Detects protocol deviations and data anomalies faster.
- KPIs: Define and track metrics such as patient retention, protocol adherence, and site responsiveness.
- Risk mitigation plans: Adjust quickly to tech failures or participant dropouts.
-
Ensure Patient-Centricity
- Accessibility: Ensure tech platforms are inclusive, user-friendly, and compatible across devices.
- Flexibility: Offer patients choice between virtual and in-person visits.
- Engagement: Use reminders, digital diaries, and personalized outreach to boost adherence.
-
Conduct Pilot Programs
- Start small: Test DCT components on a single site or cohort before scaling.
- Analyze outcomes: Assess patient satisfaction, data quality, and operational efficiency.
- Iterate and improve: Use feedback to optimize future decentralized models.
Recommendations from the FDA for Decentralized Clinical Trials
- Sponsors should engage with the FDA early in the planning process for feedback.
- Risk-based monitoring, assessment and mitigation strategies should be documented.
- Data privacy and cybersecurity measures must be top priorities.
- Informed consent must be obtained while ensuring participant understanding.
- Sponsors should establish methods to confirm participant identity remotely.
- Protocols should define procedures to ensure protocol compliance.
- Local healthcare providers should be trained to correctly perform delegated study procedures.
- Centralized monitoring and quality control are important for multisite studies.
Decentralized Clinical Trials: Challenges & Solutions
While there are many potential benefits of decentralized clinical trials (DCTs), several challenges are associated with this clinical trial model. Some of the key challenges include:
Challenge 1
Technology InfrastructureDCTs rely heavily on digital technologies, such as telemedicine, remote monitoring, and electronic data capture. Ensuring that the technology infrastructure is appropriate for the study objectives and patient population can be challenging and require significant investment in technology and training.
To overcome these challenges, sponsors should invest in scalable, user-friendly digital platforms aligned with study goals and patient needs. This includes robust training for both staff and participants and collaborates with experienced technology partners to support infrastructure, usability and compliance throughout the trial.
Alternatively, sponsors may consider partnering with a full-service Contract Research Organization (CRO) that specializes in decentralized clinical trials to manage operations efficiently while allowing internal teams (sponsors) to focus on core research and development activities.
Challenge 2
Data Privacy and SecurityDCTs require collecting, storing, and transmitting large amounts of patient data. Ensuring the privacy and security of this data can be challenging and requires strict adherence to regulatory requirements and best practices in data security.
To ensure data privacy and security in DCTs, implement robust encryption, secure data storage, and role-based access controls while maintaining compliance with regulations like HIPAA and GDPR. Regular security audits and staff training are also crucial to prevent breaches and ensure data integrity.
Challenge 3
Regulatory ComplianceDCTs are subject to the same regulatory requirements as traditional clinical trials but with additional challenges related to ensuring the quality, accuracy, and integrity of the data collected remotely. Regulatory agencies are still developing guidelines and best practices for DCTs, which can create uncertainty for trial sponsors.
To navigate regulatory challenges in DCTs, sponsors should proactively engage with regulatory authorities, follow emerging guidance, and implement rigorous remote data validation processes.
Challenge 4
Patient Engagement and RetentionWhile DCTs can make it easier for patients to participate in clinical trials, they require patients to be more self-motivated and engaged in the study. Ensuring patient retention and compliance can be challenging and requires careful patient selection and engagement strategies.
Enhance patient retention in DCTs by using intuitive digital tools, regular communication, and personalized support. Carefully select tech-savvy participants and keep them engaged through reminders and incentives.
Challenge 5
Recruitment and Retention of SitesDCTs can reduce the need for physical trial sites but still require sites to participate in the study, particularly for patient screening and monitoring. Ensuring the engagement and retention of locations can be challenging, particularly for smaller areas with limited resources.
To ensure site engagement and retention in DCTs, provide tailored support, training, and streamlined processes that reduce site burden. Also, collaborate closely with sites in resource-limited areas, offering flexible participation models and consistent communication to maintain involvement.
The Future of Decentralized Clinical Trials
Decentralized clinical trials (DCTs) can transform medical research by enabling more patient-centric, flexible, and efficient clinical trial designs. As technology continues to evolve, the future of medical research is likely to be increasingly decentralized, with more trials incorporating digital technologies and remote monitoring.
Some of the key trends shaping the future of DCTs include the following:
- Expansion of Remote Monitoring
Remote monitoring is likely to become even more prevalent in DCTs, with the continued development of wearable devices, mobile applications, and other digital tools. Remote monitoring can enable real-time data collection and analysis and can improve patient engagement and retention. - Increased Use of Artificial Intelligence (AI)
AI can transform many aspects of clinical research, from patient recruitment to data analysis. AI-powered algorithms can help to identify eligible patients, predict outcomes, and optimize trial designs, improving the efficiency and success of clinical trials. - Emphasis on Patient Engagement
Patient engagement is likely to become an even greater focus of DCTs in the future as trials increasingly incorporate patient-centric designs and digital tools. Patient engagement can improve the quality and completeness of data collection and patient satisfaction and retention.
- Expansion of Decentralized Trial Designs
Decentralized trial designs are likely to become even more common as regulators and sponsors recognize the benefits of these designs. Decentralized trials can be more flexible and adaptable than traditional trial designs, enabling more efficient and cost-effective research. - Integration of Real-World Data
Real-world data, such as data from electronic health records or claims databases, can provide valuable insights into patient outcomes and treatment patterns. Integration of real-world data into clinical trial designs can enable more personalized and effective treatments and can also reduce the burden of data collection on patients.
Conclusion
The shift toward decentralized clinical trials represents a transformative step in modern research, offering a more patient-centric, accessible, and efficient approach to data collection and execution. Successful implementation requires strategic planning, a robust decentralized clinical trials platform, and strong decentralized clinical trials technology to enhance trial accessibility, efficiency, and data quality.
Looking ahead, the future of decentralized clinical trials promises broader adoption, integration with real-world data and AI, and alignment with Decentralised Clinical Trials FDA guidance – accelerating timelines and improving outcomes for all stakeholders.

Clinvigilant Research
Clinvigilant Research is a global full-service CRO (Contract Research Organization) dedicated to advancing clinical development with scientific precision and patient-centric solutions. With our end-to-end CRO services sponsors can accelerate time-to-market while maintaining the highest standards of quality and compliance. With global reach and deep therapeutic expertise, we partners with sponsors to bring safe, effective, and innovative therapies to patients worldwide.

